If you are researching adipose tissue banking, one of the first questions on your mind is likely about safety. This guide covers every stage of the banking process, from tissue collection through long-term cryopreservation, so you can evaluate the risks and benefits with confidence. By the end, you will understand what validated safety protocols look like, what complication rates the research shows, and what questions to ask before moving forward.
TLDR: Adipose tissue collection is a minimally invasive outpatient procedure with complication rates well below 1% in peer-reviewed studies. Validated cryopreservation protocols maintain cell viability for years, and clinical trials consistently report favorable safety profiles with few serious adverse events. However, banking tissue does not guarantee future treatment access or clinical benefit, and all medical decisions should involve your physician.
Important Disclaimer: Save My Fat does not provide FDA-approved treatments or cures for any disease. Banking adipose tissue today does not guarantee eligibility, access, or clinical benefit from any future therapy, clinical trial, or medical program. All content is for educational purposes only and does not constitute medical advice. Patients must consult their own licensed healthcare professionals regarding all medical decisions.
You have probably heard about adipose tissue banking and wondered whether the process is truly safe. Maybe you are drawn to the idea of preserving your own biological material for potential future use, but the thought of any medical procedure raises natural concerns. Questions like “What are the real risks?” and “How do I know my tissue will remain viable?” deserve honest, evidence-based answers.
This guide breaks down safety at every step: the collection procedure, cryopreservation, long-term storage standards, and what clinical research reveals about adverse events. You will also find a comparison to other common medical procedures for context, along with practical steps to evaluate any tissue banking provider.
Overview: What Does “Safe” Mean in Tissue Banking?
When evaluating the safety of adipose tissue banking, it helps to consider three distinct phases. First is the tissue collection procedure itself, typically a form of liposuction performed under local anesthesia. Second is the processing and cryopreservation, where tissue is prepared and frozen using validated protocols. Third is long-term storage in controlled facilities. Each phase carries its own risk profile, and the published research addresses each one.
It is also important to distinguish between the safety of the banking process and the outcomes of any future use. This article focuses specifically on whether the collection, preservation, and storage of adipose tissue is safe. Questions about future applications involve separate regulatory and scientific considerations covered in our emerging research resources.
Safety of the Tissue Collection Procedure
Adipose tissue is collected through a minimally invasive liposuction procedure, typically performed under local (tumescent) anesthesia in an outpatient setting. For tissue banking purposes, the volume collected is relatively small, usually 50 to 200 mL, far less than cosmetic liposuction procedures.
What the Research Shows
Large-scale safety analyses paint a reassuring picture. A 2024 systematic review found that infection rates averaged just 0.020% and venous thromboembolism (VTE) occurred in only 0.017% of cases (Ghaffarpasand et al., 2024). A national analysis of over 4,800 liposuction cases reported overall complication rates between 0.40% and 0.63%, with the vast majority being minor and self-limiting (Jarmusch et al., 2024). Even studies examining high-definition liposuction, which is more aggressive than tissue banking collection, found major complication rates of only 0.2% to 0.7% (Prendergast et al., 2023) (Kaoutzanis et al., 2017).
What to Expect During Recovery
Most patients return to normal activities within one to three days. Common experiences include mild soreness, minor bruising, and temporary swelling at the collection site. These are self-limiting and typically managed with over-the-counter pain relief. The tumescent anesthesia technique has been shown to provide effective pain control while minimizing bleeding risk (Klein, 2000).
| Complication Type | Reported Rate | Context |
|---|---|---|
| Minor complications (bruising, swelling, soreness) | Common, self-limiting | Expected part of recovery |
| Infection | 0.020% to 0.1% | Lower than many routine procedures |
| Major complications | 0.2% to 0.7% | Includes all liposuction types |
| Venous thromboembolism | 0.017% to 0.06% | Comparable to minor surgeries |
| Mortality | Extremely rare | Not reported in small-volume collection studies |
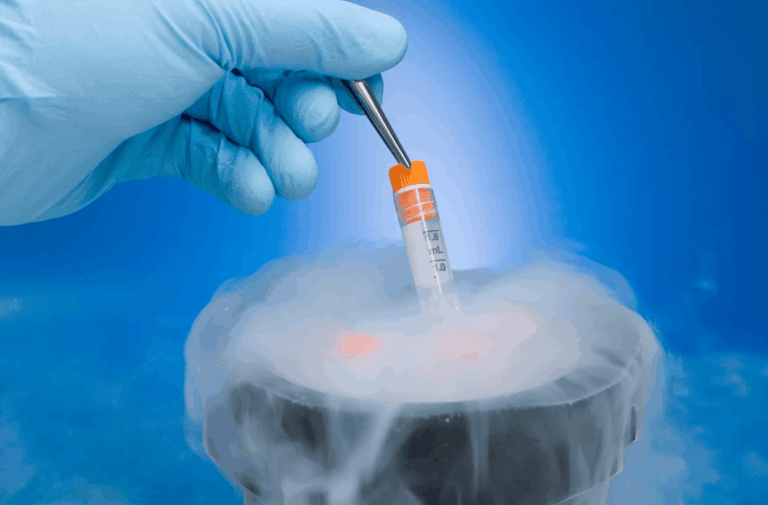
Cryopreservation: How Tissue Is Safely Preserved
Once adipose tissue is collected, it must be properly processed and frozen to maintain cellular viability. Cryopreservation uses controlled-rate freezing protocols and cryoprotective agents to prevent ice crystal damage to cells during the transition to ultra-low temperatures.
Validated Protocols and Cell Viability
Research consistently shows that validated cryopreservation protocols preserve the key biological characteristics of adipose-derived cells. A study published in Cryobiology found that cryopreserved cells maintained their surface marker expression, proliferation capacity, and differentiation ability with no significant differences from fresh cells (Gonda et al., 2008).
Longer-term studies are equally encouraging. Researchers have demonstrated successful cell isolation from adipose tissue cryopreserved for over three years, with cells retaining proliferative ability and stem cell markers (Minonzio et al., 2014). Additional research confirms that long-term cryopreservation maintains viability at levels suitable for potential clinical applications (Devitt et al., 2015). Notably, comparative research suggests adipose-derived cells may be more robust during cryopreservation than cells from other sources such as bone marrow (Davies et al., 2014).
Clinical-grade cryopreservation follows strict quality control protocols. A study examining GMP-compliant processing confirmed that standardized approaches reliably produce cells meeting benchmarks for viability, sterility, and biological function (Laloze et al., 2020).
| Safety Protocol | Purpose | Standard |
|---|---|---|
| Controlled-rate freezing | Prevents damaging ice crystal formation | Cooling at negative 1 degree Celsius per minute |
| Cryoprotective agents (DMSO) | Protects cells during freezing | 5% to 10% concentration, validated formulations |
| Sterility testing | Ensures no bacterial or fungal contamination | Negative cultures required before and after processing |
| Viability assessment | Confirms cells survived preservation | Target greater than 80% viability post-thaw |
| Mycoplasma screening | Detects common contaminant | Negative result required for release |
Storage Facility Standards and Oversight
Long-term storage of cryopreserved tissue requires specialized infrastructure and ongoing quality control. Tissue banking facilities that handle human cells and tissues must comply with FDA requirements under 21 CFR Part 1271, which establishes standards for donor screening, processing, storage, and adverse event reporting.
Cryopreserved tissue is stored in liquid nitrogen vapor-phase systems at temperatures around negative 150 to negative 196 degrees Celsius. At these temperatures, biological activity is effectively suspended indefinitely when conditions are maintained. Facilities implement continuous temperature monitoring, backup systems, and documented protocols. Save My Fat works with facilities that follow Current Good Tissue Practice (CGTP) standards. Learn more about how stem cell banking works on our process page.
Tissue banking establishments must register with the FDA and comply with federal HCT/P regulations, including donor screening, adverse reaction reporting, and facility inspections. The FDA maintains a searchable database of registered tissue establishments for patient verification.
Potential Risks and Honest Limitations
No medical procedure is entirely without risk. The primary risks associated with adipose tissue collection include infection, bleeding, bruising, and temporary discomfort. Patients with bleeding disorders, active infections, or blood thinners should discuss additional considerations with their physician.
While validated cryopreservation protocols maintain cell viability at high rates, some cell loss during the freeze-thaw cycle is expected, typically 10% to 20%. Data on preservation beyond a decade is still accumulating.
Most importantly, banking preserves biological material for potential future use but does not guarantee any specific clinical outcome. Future use depends on the regulatory status of therapies at that time, clinical trial eligibility, physician recommendations, and product availability. Save My Fat is transparent about these limitations, as outlined in our disclaimer.
How Does This Compare to Other Procedures?
Putting adipose tissue collection risks into perspective helps with decision-making.
| Procedure | Major Complication Rate | Context |
|---|---|---|
| Adipose tissue collection (small-volume liposuction) | 0.2% to 0.7% | Outpatient, local anesthesia |
| Dental extraction (wisdom teeth) | 1% to 5% | Common oral surgery |
| Bone marrow aspiration | 0.5% to 6% | Alternative stem cell source |
| Knee arthroscopy | 0.5% to 2% | Common orthopedic procedure |
| Colonoscopy | 0.1% to 0.3% | Routine screening procedure |
The comparison to bone marrow aspiration is particularly relevant. Bone marrow harvesting requires needle insertion through bone under general or spinal anesthesia, with 70% of patients reporting post-procedure pain and 32% reporting severe pain. Adipose tissue collection involves significantly less discomfort and faster recovery.
Long-Term Clinical Safety Evidence
The broader clinical safety profile of adipose-derived cells has been extensively studied. A systematic review of adverse events across clinical trials found a favorable safety profile, with serious adverse events being rare and generally unrelated to the cells themselves (Toyserkani et al., 2017). The most commonly reported adverse event was mild, temporary discomfort at the collection site (Berman et al., 2018).
Studies involving allogeneic (donor-derived) adipose stem cells have also shown the therapy to be well tolerated (Aung et al., 2025), and research in elderly populations has confirmed safety and tolerability (Rao et al., 2024). Across thousands of documented procedures, zero cases of teratoma (tumor) formation have been reported with adipose-derived stem cells, a critical safety distinction from other cell types. You can explore related topics on our stem cell research areas page.
How to Evaluate a Tissue Banking Provider
Making an informed decision involves asking the right questions.
Step 1: Verify FDA compliance. Ask whether the storage facility is registered with the FDA and complies with 21 CFR Part 1271.
Step 2: Ask about processing protocols. Request details about cryopreservation methods and quality control testing (viability, sterility, mycoplasma).
Step 3: Understand the physician’s role. The tissue collection procedure should be performed by a qualified, licensed physician.
Step 4: Review realistic expectations. A trustworthy provider will be upfront about what banking does and does not guarantee. Be cautious of any company promising future cures.
Step 5: Consult your own physician. Discuss tissue banking with your primary care doctor or specialist to evaluate whether banking aligns with your health planning goals.
Save My Fat’s About Us page outlines our commitment to transparency, and our patient resources provide additional information.
Frequently Asked Questions About Banking Safety
Q: How safe is the tissue collection procedure? A: Published research shows major complication rates of 0.2% to 0.7%, with infection rates around 0.020% to 0.1%. The procedure is performed under local anesthesia in an outpatient setting, and most patients return to normal activities within one to three days. Discuss individual risk factors with your physician beforehand.
Q: Will my tissue remain viable in long-term storage? A: Peer-reviewed studies confirm that validated cryopreservation protocols maintain cell viability, surface marker expression, and biological function after years of storage. Research has demonstrated successful cell recovery from tissue stored for over three years, and ultra-low temperatures effectively suspend biological activity indefinitely when conditions are maintained.
Q: Are there any long-term risks from banking tissue? A: The storage of tissue in a cryopreservation facility has no ongoing health risks to you. The collection procedure carries minor, short-term risks described above, but once tissue is banked, there are no continued physical risks to the patient.
Q: Does banking my tissue mean I can get stem cell treatment? A: No. Banking preserves your tissue for potential future opportunities, but it does not guarantee access to any therapy, clinical trial, or medical program. Future use depends on evolving science, FDA regulatory decisions, clinical eligibility, and physician recommendations. Learn more through our emerging research page.
Q: How is this different from clinics offering immediate stem cell treatments? A: Save My Fat is a tissue banking service focused on preservation for potential future use through legitimate FDA-regulated pathways. This is fundamentally different from clinics marketing unapproved treatments for specific diseases. The FDA’s consumer alert on regenerative medicine provides guidance on identifying compliant services.
Key Takeaways
Tissue Collection Safety
- Small-volume liposuction has major complication rates below 1%
- Infection rates average 0.020% to 0.1% in published literature
- Local anesthesia, small incisions, and one to three day recovery
Cryopreservation Reliability
- Validated protocols preserve cell viability, markers, and biological function
- Successful cell recovery demonstrated after years of storage
- Adipose-derived cells show resilience during the freeze-thaw process
Clinical Evidence Base
- Systematic reviews report favorable safety profiles across trials
- Zero teratoma formations reported across thousands of procedures
- Safety confirmed in elderly and comorbid patient populations
Important Limitations
- Some cell loss (10% to 20%) during freeze-thaw is expected
- Banking does not guarantee future treatment access or clinical benefit
- All medical decisions should involve a licensed physician
Ready to Learn More About Adipose Tissue Banking?
You now have an evidence-based understanding of the safety profile at every stage of adipose tissue banking. The research consistently shows that tissue collection is a low-risk outpatient procedure, validated cryopreservation maintains cell viability, and clinical safety data is favorable.
Save My Fat helps individuals preserve adipose tissue through validated cryopreservation protocols for potential future use in FDA-regulated pathways. We prioritize transparency, regulatory compliance, and honest communication about both possibilities and limitations.
Banked tissue may also be accessible to first-degree family members under certain guidelines, making banking a consideration for your broader family health planning.
Ready to learn more?
Visit: https://savemyfat.com Contact Us: https://savemyfat.com/contact-us/
Save My Fat Adipose Tissue Banking for Future Regenerative Medicine Opportunities
Last updated: February 16, 2026